Heat Biologics is an Opportunity Among Soaring Immunotherapy Biotech Valuations
NEW YORK, NY / ACCESSWIRE / April 14, 2015 / Vaccination, in medical terminology, is injection of a disease-causing microorganism that stimulates the immune system to fight against infection, using the body’s own defense mechanisms. Vaccination has all but wiped out polio, tetanus, chicken pox and measles with non-live proteins that lead to an antibody response to promote internal, natural healing. A new type of immunotherapy has emerged in recent years, led by Heat Biologics, Inc. (HTBX: NASDAQ), using live cellular material to cause an immune reaction with a more potent form of white blood cells that seek out and destroy cancer.
Ways to fight cancer has grabbed attention from press – CBS ’60 Minutes’ recently viewed a program for new ways to fight the disease to much applause by viewers. A three-part documentary, first aired last week on PBS, chronicles patients’ battle with malignancies, based on the Pulitzer Prize winning book ‘Cancer’ by Siddhartha Mukherjee.
While I hesitate to term this ‘hype’, fact remains that valuations for cancer-killing drug companies have increased, as I describe later. In this article, I will also explore the adverse effects these same drugs have on patients, which is very similar to chemotherapy, an unpleasant inhibitor of quality of life. Heat Biologics, however, has not shown such side effects, only pain at the injection site, as explained below.
Chimeric antigen receptor (CAR) T-cell therapy is the cancer immunotherapy of choice among Big Pharma where the patient’s own T cells are isolated in the lab, blended with a synthetic receptor to recognize a particular antigen or protein, and re-infused into the patient to bind with cancer cells and kill them. Scientific enthusiasm started around 2011 when investigators at the University of Pennsylvania published work on three patients whose chronic lymphocytic leukemia showed remission after only one dose of CAR-T therapy. Memorial Sloan Kettering and the National Cancer Institute jumped on board, both respectable institutions happy to take the time to explore what these new therapies can do.
The technology, however, is expensive, time consuming, and fraught with side effects. CAR-T drugs carry with them a “worrisome” side effect, according to researchers at Children’s Hospital of Pennsylvania, the most common being cytokine-release syndrome, leading to high fever and dangerous drops in blood pressure. Even mild side effects must be managed with steroids, a treatment with a limited time frame.
Heat Biologics’ technology, based on heat-shock proteins, can be applied to any type of cancer. Treatment does not involve extracting and processing the patient’s own cells; because of this it is far less expensive to make – a special drug created for an individual patient is not necessary, contrary to Dendreon Corporation (DNDN:NASDAQ), the grandfather of cancer vaccines whose high cost of manufacturing put the company in bankruptcy.
New and more evolved, Heat Biologics’ science rests on its patented Immune Pan-Antigen Cytotoxic Therapy (ImPACT), a ‘helper’ remedy specific to CD8+ cytotoxic T-cell immune responses seen as essential in today’s world of oncology immunotherapies. In Heat Biologic’s laboratory, cancer cell lines are genetically engineered to express a naturally-occurring heat-shock, or stress protein called gp96.
Living cancer cells, through ImPACT technology, take gp96, bound to all human cells by an amino acid sequence known as KDEL that, when cleaved by ImPACT, transforms cells into microscopic ‘pumps’ that release gp96 (and antigens) over a period of time to activate the immune system against a wide range of cancer pathologies for a therapeutic result. The vaccine is comprised of allogenic living cells, meaning they do not come from the patient – a major advantage over current cancer immunotherapies and less prone to side effects. Heat’s Phase II trials are in non-small cell lung cancer (NSCLC) and bladder cancer, two pervasive and mortal conditions often leading to metastases.
My conversations with Jeffrey Wolf, founder, Chairman and CEO of Heat Biologics, sums up this innovative scientific tool – cells are reprogrammed to pinpoint a broad spectrum of cancer-associated antigens that coupled with gp96 create an immune response by mobilizing killer T-cells, that in turn target multiple cancers, recognized as the disease that they indeed are, summoning the patient’s immune system to fight back.
The basis of this technology, heat shock proteins (HSPs), fall into three categories depending on molecular weight and were first identified as cellular responses to environmental stress like infection, inflammation, exposure to toxins or metabolic disruption from food, water and oxygen deprivation. Main functions include protecting tissue from pathogens (disease) by stimulating the immune system to action, facilitating correct protein configuration within the nucleus of the cell to avoid mutation, and disposal of bad proteins that can lead to genetic disease.
Heat Biologic’s solid patent estate covers the use of HSPs in a vaccine using gp96-associated peptides (components of proteins) biochemically cross-linked with CD8 cells via dendritic cells that work to take antigen material and place it on the surface of immune system T-cells; in other words, the company created an effective messenger to combat cancer. It was discovered as early as 1988 that HSPs ‘chaperone’ antigens to immune system cells, in addition to making sure transfer and assembly of proteins is done correctly in normal cells, lending credence to Heat Biologic’s technology.
Live, genetically-engineered cells subject to radiation prevent unnecessary multiplication and can secrete the gp96 heat shock protein/antigen cocktail for up to 10 days, and this ability has shown value in clinical trials. Method patents cover commercial production of a large number of HSPs conveniently and rapidly, when only a small sample of cancer is provided. A patent has been granted in Australia, with several pending in the US, Canada, Europe, Israel, China, India, Hong Kong, South Korea and Japan; terms extend to 2029.
Much of Heat Biologics work in HSPs is attributed to the chairman of its Scientific and Clinical Advisory Board, Eckhard Podack, M.D, Ph.D., serving as department head of microbiology at the University of Miami, Miller School of Medicine, whose early accomplishments included discovery of perforin, a protein found in T-cytotoxic lymphocytes and natural killer cells, two important components of the human immune system, that helps bust apart diseased cells to stimulate the immune response to fight disease. His later work involved development of HSPs to create cell-based vaccines for effective anti-tumor activity and has become the driving force behind Heat Biologics’ technology which so far has shown clinical success through the creation of a vaccine that significantly retards tumor growth rate.
Other biotech firms have taken notice. Last February, OncoSec Medical Inc. (ONCS:OTCQB) agreed to jointly evaluate its ImmunoPulse intratumoral DNA platform delivery of Interlueken-12 in Phase II trials with Heat Biologic’s gp96-based ImPact immunotherapy. OncoSec Medical’s use of electroporation, the process by which an electrical field applied to cells increases the permeability of a cell’s membrane, allows drugs or DNA to be introduced more easily. This could greatly enhance Heat Biologics technology, and a variety of cancers are expected to be explored.
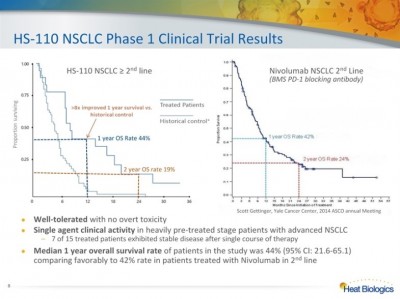
HS-110 is Heat Biologic’s drug for non-small cell lung cancer (NSCLC), a lethal disease accounting for almost 30% of all cancer fatalities per annum – dwarfing deaths from colon, breast and prostate cancers combined. Heat’s Phase I, started in March 2013, is a single institution study of 18 patients that showed good safety and significant control of disease. Of 15 patients, 11 (73.3%) responded well to HS-110 vaccination with high levels of immune response, no overt toxicity, and a median overall survival rate of 44% versus a 42% survival with Opdivo, a new cancer immunotherapy by Bristol-Myers Squibb (BMY:NYSE). Median survival time of HS-110 treated patients was 16.5 months, 275x higher than those with no appreciable immune response (due mostly to overtreatment with chemotherapy regimens). These positive results led to Phase II studies to further show HS-110 as a therapeutic vaccination can delay tumor growth and extend survival.
Heat Biologic’s HS-110 Phase II protocol consists of a lung cancer line, genetically modified using ImPACT technology, in combination with cyclophosphamide (a cancer treatment) and chemotherapy to allow a wide secretion of lung cancer antigens, with gp96, to elicit T-cell immune response. The study design of approximately 120 patients in 20-30 clinical sites contains a group receiving only chemotherapy for a direct comparison. Patient enrollment is planned for subjects who have failed other treatments, a bold clinical move for Heat Biologics. Endpoints for this study include overall survival, progression-free survival and immune response at six and 12 months through biopsy tissue analysis to show expression of antigens through T-cells – proof of concept for the drug. Enrollment should complete in the second half of 2015.
Another Phase II clinical trial uses Heat Biologics’ second compound, HS-410, in non-muscle invasive bladder cancer (NMIBC), fast-tracked at FDA, where the only available therapeutic development for this deadly disease – bacilli Calmette Guerin (BCG), an immunotherapy taken from cow tuberculosis, emerged nearly 40 years ago and still used today. Bladder cancer is notorious for reappearance with rates up to 70%, thus BCG has become the maintenance drug of choice to repress reoccurence. However, prevention of disease progression with BCG as ongoing therapy is debatable among urologists, given its high side effect profile coupled with an unclear mechanism of action. Patients with impaired immune systems, regardless of cause, cannot receive BCG – a medical Catch-22 since such this older immunotherapy carries with it high fever, pneumonia, hepatitis, circulatory collapse and organ dysfunction.
Bladder cancer, representing almost 5% of all new cancer cases in the US, affects 74,690 people per year and has a prevalence of approximately 571,000 people today living with the disease. Cancer.gov pegs annualized net costs of bladder cancer care (including initial, continuing, and last year of life), using 2010 numbers for people over 65, at $103,820 for women and $101,409 for men, making it perhaps the most expensive malignancy in America.
Heat Biologics’ Phase II, randomized, placebo-controlled and double-blind incorporates roughly 100 patients and comprise several arms: low dose HS-410 with BCG; high dose HS-410 with BCG, placebo plus BCG, and HS-410 as a monotherapy. The last arm is vitally important to show HS-410 as a monotherapy for bladder cancer and may, if successful, direct the design of a pivotal trial to possibly introduce to the medical community HS-410 as a replacement for BCG – a revolutionary step toward treatment of this expensive and prevalent disease. Patient enrollment is expected to complete by 3Q2015.
Immunotherapy in bladder cancer is spotty; hence, competition is light. Montreal-based Telesta Therapeutics Inc. (TST.TO: Toronto) plans to file a product similar to BCG with the FDA, but the agency has postponed approval due to manufacturing issues, extending decisions until June. Spectrum Pharmaceuticals, Inc. (SPPI:NASDAQ) wants to file an application for EOquin this year for bladder cancer but the drug failed primary endpoints in two previous trials; as an analog of mitomycin C, an antiquated treatment similar to BCG, it is not expected to be accepted by the medical community because side effects mimic chemotherapy.
Yet Cellectis SA (NASDAQ:CLLS), a French company starting Phase I trials of a CAR-T drug for blood cancers recently raised $228.3 million and listed in the US, backed by heavy weight underwriters. Market capitalization is $1.18 billion. This on the heels of an $80 million up-front payment last June from Pfizer, Inc. (PFE:NYSE).
Merck & Co. (MRK:NYSE), not to be left out, received approval for KEYTRUDA, a cancer immunotherapy called anti-PD-1, causing programmed cell death to tumors. First indication is for skin cancer after other treatments, including monoclonal antibodies, have failed. However, this new wonder drug is suspected to attack otherwise healthy organs and tissues, in a life-threatening manner. Side effects include pneumonia, intestinal holes, hepatitis, rapid heartbeat, hair loss, fainting, severe muscle weakness, and kidney failure. Don’t even think about getting pregnant. Merck plans to test KEYTRUDA in lung cancer in the second half of next year in conjunction with privately-held Syndax Pharmaceuticals Inc.’s monoclonal antibody compound that has shown some efficacy in mice for said indication, but is best known for its Phase III work in hormone replacement therapy in post-menopausal women.
A confusing cross-therapy indication.
Much money has changed hands in the realm of cancer immunotherapy. Juno Therapeutics Inc. (JUNO:NASDAQ), boasting a CAR-T drug, raised $265 million in an IPO at the end of last year. Bellicum Pharmaceuticals, Inc. (BLCM:NASDAQ), only in Phase I/II trials for blood cancers with CAR-T technology and Phase I for prostate, went public at $140 million. Kite Pharma, Inc. (KITE:NASDAQ), another recent public offering, trades at a $2.3 billion market capitalization, based on its early-stage CAR-T therapy.

Valuations for cancer immunotherpies are soaring. In 2014, almost 90 biotechs reached the public markets raising nearly $6 billion. Some of the companies mentioned in this article are represented in the chart below, and, as any investor can see, Heat Biologics is far down the scale, despite two Phase II trials in cancer immunotherapy, with a market cap of only $51.4 million.
Risks to investing in Heat Biologics include the usual hazards for mid-stage biotechs but most important is whether Phase II positive results emerge in late stage trials. Cancer immunotherapies, as described above, have side effects but adverse events for both HS-110 and HS-410 have shown only pain at the injection site. Endpoints may not be met, especially overall survival for bladder cancer, monitored for three years, a long time-frame. However, the company has been successful in a recent money raise of $12 million for working capital, clinical trials and FDA expenses, a good testimony for its science and its future potential. Along with $14.4 million in cash and equivalents as of their fiscal year end (December 31, 2014), and an operating burn rate of approximately $12 million per year, this gives Heat Biologics about two years of runway, a tight timeframe to produce clinical results that may interest a partner. Investors should also consider that Heat Biologics, unlike typical biotechs, carries long-term debt of $2.3 million.
As described above, Heat Biologics is an undervalued player in cancer immunotherapy, a hot space dominated by CAR-T treatment offered by large drug companies seeking an answer to the world’s most insidious diseases. Big Pharma, to date, has pursued immunotherapy that carries a potential high cost, to the detriment of patients via serious side effects. By contrast, Heat Biologics offers a unique vaccine that can be used across a wide patient population, just like vaccines that have successfully eradicated many diseases of the past, from polio to chicken-pox. In Phase II trials, Heat Biologics’ vaccine therapy shows a strong immune response without side effects seen in other drug trials. This alone should give them a much higher market capitalization than what is reflected in current prices.
Media contact for Small Cap Forecasting Inc.
Janet Vasquez/JVPR NY
jvasquez@jvpublicrelationsny.com
VOICE: Small Cap Forecasting Inc.
ReleaseID: 427786