Advanced Accelerator Applications Annonce Une Hausse De Plus De 24% De Ses Ventes Pour Le Premier Trimestre 2015
The AAA Receives Fast Track Designation From (Expedited Procedure) From The FDA For The Lutathera (R) In The Treatment Neuroendocrine Tumors In The Middle intestine
SAINT GENIS POUILLY, FRANCE / ACCESSWIRE / Juin 15, 2015 / Advanced Accelerator Applications SA (AAA), an international company specializing in Molecular Nuclear Medicine (MNM) announced its financial results for the first quarter 2015.
Key points
Sales up 24.5% yoy to Q1 2015 compared to Q1 2014
Designation “Fast Track” (accelerated review procedure) by US regulatory agencies (FDA) for the Lutathera (R) in the treatment of patients with carcinoid tumors of the midgut, well-differentiated metastatic or unresectable stage overexpressing of somatostatin receptors.
Obtaining a Temporary Authorisation for Use in France cohort (cohort ATU called) for Lutathera (R) in the treatment of neuroendocrine tumors (TNES)
Authorization Lutathera (R) for compassionate use in Denmark
Recruitment of the first patient in the clinical Phase I / IIa with Annexin V-128 in patients with rheumatoid arthritis and ankylosing spondylitis.
Signed a distribution agreement with Lantheus Medical Imaging, Inc. for NEUROLITE (R) (kit for the preparation of injectable solution of (99mTc) – bicisate) in France and Spain.
“I am pleased to announce that the results of AAA meet or exceed our goals, both financially and clinically. AAA continues to optimize its production processes , logistical and financial, while we prepare for the international launch of Lutathera (R) ,” said Stefano Buono, CEO of AAA.
“We believe the Lutathera (R) could have a significant impact on the way physicians treat patients with TNES overexpressing somatostatin receptors in the near future. Having obtained this accelerated review procedure FDA is an important step in expanding treatment options for these patients and highlights the significant potential impact of nuclear medicine in this new therapeutic model. We expect that the results show a significant improvement on the Clinical and meaningful level in terms of progression-free survival in patients treated with Lutathera (R),” says Stefano Buono.
Financial Results – Q1 2015
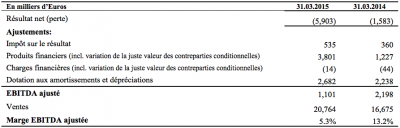
Total sales in the first quarter 2015 amounted to € 20.8 million, representing an increase of 24.5% year on year compared with € 16.7 million in Q1 2014.
For the first quarter ended March 31, 2015, the operating loss amounted to € 1.6 million, compared to € 40K for the same period in 2014.
The net loss for the first quarter 2015 amounted to € 5.9 million against a net loss of € 1.6 million euros in the first quarter 2014, an increase of the net loss of € 4.3 million.
Adjusted EBITDA for the first quarter ended March 31, 2015 amounted to € 1.1 million compared to € 2.2 million euros in Q1 2014, a decrease of € 1.1 million euros.
March 31, 2015, cash and operating cash equivalent amounted to € 39.1 million.
Designation “Fast Track” (accelerated review procedure)
AAA has received Fast Track designation (accelerated procedure) to the FDA for Lutathera (R) in the treatment of neuroendocrine tumors of the midgut. The new drug application (NDA) will also provide results of previous trials gastroenteropancreatic neuroendocrine tumors (NET-GePS).
The Lutathera (R) is a new drug currently in clinical Phase III pivotal study for the treatment of neuroendocrine tumors (TNES) well-differentiated midgut (jejunum, ileum, appendix and ascending part of the colon) in metastatic or inoperable. The Lutathera (R) selectively target somatostatin receptors, overexpressed by TNES.
Approximately 80% of all neuroendocrine tumors overexpress somatostatin receptor (particularly SSTR2 receptors). The Lutathera (R) is a somatostatin analogue radiolabeled which has a very high affinity for SSTR2. Its mechanism of action is to release radiation (high energy electrons) after internalization via SSTR2. A full treatment consists of four injections at intervals of 6 to 8 weeks.
The Lutathera (R) is currently in clinical Phase III pivotal study for the treatment of neuroendocrine tumors of the midgut in 51 clinical centers in the United States and Europe (the clinical study Netter-1). Recruitment was completed in February 2015 and 74 events of progression are required to reach the primary endpoint. The results of this study will be presented in September 2015 at the Congress of Vienna ESMO.
About Advanced Accelerator Applications
Advanced Accelerator Applications (AAA), radiopharmaceutical group founded in 2002, has extensive experience in the development of innovative products and applications for therapeutic and diagnostic and focuses particularly on the fields of molecular imaging and personalized medicine. To date, AAA has 17 production laboratories and R&D and employs over 340 employees in 11 countries (France, Italy, UK, Germany, Switzerland, Spain, Poland, Portugal, Israel, USA and Canada). In 2014, sales of AAA amounted to € 69.9 million euros (+ 29.8% vs. 2013). For more information about AAA, please visit www.adacap.com.
About Lutathera (R) and clinical trials
Lutathera (R) (or 177 Lu-DOTATATE) is a peptide analogue of somatostatin labeled with Lu-177 is currently under development for the treatment of neuroendocrine tumors gastro entero-pancreatic (GEP-NETs). This new drug has received the orphan drug designation from the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) in the USA. This status grants the Lutathera (R) an extension of the protection of postmarketing patent for a period of 7 years in the US and 10 years in Europe. The Lutathera (R) is currently approved in compassionate use in ten European countries where no alternative therapy is available and with special permission.
Lutathera (R) belongs to an emerging family of treatments called peptide receptor radionuclide therapy (PRRT) targeting carcinoid tumors using somatostatin analog peptides radiolabeled.
There is a real lack in the therapeutic arsenal to effectively treat inoperable advanced NETs. Indeed there is currently no approved treatment for pancreatic NETs other than (about 10% of NET of pancreatic origin are) progressing under somatostatin analogues. Currently in clinical phase III study with the pivotal clinical study NETTER-1, Lutathera (R) is the most advanced candidate in development PRRT.
Netter-1 is a Phase III, multicenter, international, randomized, parallel group, controlled by a reference treatment, to assess the efficacy and safety of Lutathera (R) compared with a double dose of “Octreotide LAR in patients with carcinoid tumors of the midgut (TNES midgut) inoperable, which overexpress somatostatin receptors and progress under treatment with Octreotide LAR. The main criterion is the assessment of progression-free survival (PFS). Secondary endpoints include safety, objective response rate, time to tumor progression, overall survival and quality of life. The study is conducted in 51 clinical centers in the United States and Europe. Recruitment was completed in February 2015 and 74 events of progression are required to reach the primary endpoint. Lutathera (R) aims to address a void in the therapeutic arsenal, because after progression under similar “cold” somatostatin such as Octreotide LAR (Novartis) or Somatuline (Ipsen), there are no alternative treatments approved in this indication.
About Molecular Nuclear Medicine (MNM)
Molecular Nuclear Medicine (MNM) is a medical specialty that uses very small amounts of active substances, called radiopharmaceuticals, to create images of organs and lesions and treat a number of diseases, such as cancer. Radiopharmaceuticals are injected into the body and bind to targeted on selected organs or lesions to reveal specific biochemical processes. The Nuclear Molecular Diagnostics allows physicians to accurately diagnose complex diseases such as cancer, cardiovascular disease and neurological disorders at early stages and to improve monitoring. Patients are injected radiopharmaceutical tracer and images with PET cameras (Positron Emission Tomography) or SPECT (Positron Single Photon Emission) are produced.
*EBITDA Reconciliation to net income (loss) for the first quarter of 2015 for continuing operations
Discharge
This press release may contain forward-looking statements. All statements, other than statements of historical facts, included in this press release, including statements regarding the company’s strategy, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipates,” “believes,” “estimates,” “expects,” “intends,” “may,” “plans,” “expects,” “plans,” “target,” ” potential ,” ” will ,” ” would,” ” could,” ” should,” ” continue “and similar expressions are intended to identify forward-looking statements, although some forward-looking statements do not contain these specific words. Forward-looking statements reflect the current expectations of the Company regarding future events. These forward-looking statements involve risks and uncertainties and other factors that could cause actual results to significantly differ from future results, performance or achievements expressed or implied by such statements. These factors include, but are not limited to, changing market conditions, the successful and timely completion of clinical studies, the approval of the EMA, the FDA and other regulatory approvals for our products in development , the establishment of corporate alliances, the impact of competition in terms of products and prices, product development and uncertainties related to the regulatory approval process or the ability to obtain pharmaceutical products in quantity sufficient or acceptable standards for regulatory health authorities to complete clinical trials or to meet commercial demand. AAA is providing the information in this press release to this date and is not required to update the forward-looking statements as a result of new information, future events or otherwise, unless laws applicable securities require.
Contacts :
AAA Press Relations
Laetitia Defaye
Head of Corporate Communications
laetitia.defaye@adacap.com
Tel: +33 (0) 6 86 65 73 52
Véronique Mermet
Communications Officer
info@adacap.com
Tel: +33 (0) 4 50 99 30 70
AAA Investor Relations
Jordan Silverstein
Director of Investor Relations
jordan.silverstein@adacap.com
Tel: + 1-212-235-2394
Contact Media
FTI Consulting (UK)
Julia Phillips Julia.Phillips@fticonsulting.com
Tel: +44 (0) 203 727 1000
Natalie Garland Collins
Natalie.Garland-Collins@fticonsulting.com
Tel: +44 (0) 203 727 1000
iCorporate (Italy)
Elisa Piacentino
elisa.piacentino@icorporate.it
Tel: +39 02 4678754-39 366 9134595
JV Public Relations NY (US)
Janet Vasquez
jvasquez@jvprny.com
Tel: + 1-212- 645-5498 – + 1-917- 569-7470
SOURCE: Advanced Accelerator Applications
ReleaseID: 429820