Ceruvia Lifesciences Drug Trials Work To Turn Psychedelic Research Into Medicine for OCD, Headache Disorders
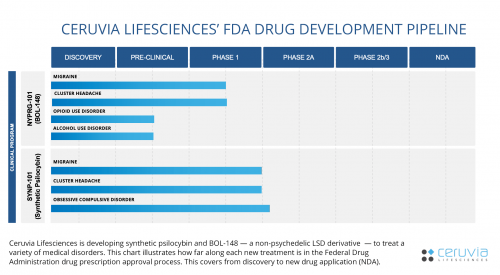
Ceruvia Lifesciences is pursuing groundbreaking clinical trials to treat conditions such as migraine, cluster headache and obsessive-compulsive disorder. Synthetic psilocybin and BOL-148 — a non-psychedelic LSD derivative — appear to produce better results than current treatments.
Greenwich, Conn., United States – August 6, 2021 /MarketersMedia/ —
Ceruvia Lifesciences is channeling funding into clinical trials for difficult-to-treat conditions with great unmet need including headache disorders and OCD.
Ceruvia Lifesciences (formerly CH-TAC) is pursuing FDA approval for these new treatments.
Ceruvia Lifesciences is the first to produce LSD and BOL-148 with manufacturing processes adhering to the highest standards required by the European Union and US regulatory agencies.
The company is supplying academic research sites with free LSD to support clinical research. Psilocybin and LSD have been shown to be safe when administered in a therapeutic setting.
Since 2015, Yale University has been the site of Ceruvia Lifesciences’ research to treat disorders including cluster headache, migraine and OCD. The university is key to advancing the research. Ceruvia Lifescience has established additional collaborations with Harvard Medical School, New York University School of Medicine, Usona Institute, Heffter Research Institute and Clusterbusters, a nonprofit for cluster headache sufferers.
Ceruvia Lifesciences’ Founder and CEO Carey Turnbull has been working to advance psychedelic research for more than a decade. Turnbull, as a fundraiser and donor, has contributed more than $30 million to psychedelic research. He is a board member of the Usona Institute and President of the Heffter Research Institute, and has worked in commercial early-stage drug development for half a dozen companies variously as an investor, founder, and CEO since 2010.
He also co-founded B.More, a company pursuing groundbreaking clinical trials to treat alcoholism with synthetic psilocybin, and Freedom to Operate, a nonprofit protecting psychedelic science and medical development for public benefit.
Ceruvia Lifesciences is initiating Phase 2 clinical trials of the FDA prescription approval process to evaluate the effectiveness of synthetic psilocybin on OCD. This could bring relief to the 3.9 million patients diagnosed with OCD in the US cited by the National Institute of Mental Health.
The compounds have shown promising results in early clinical trials. Unlike traditional medicines prescribed to treat mental disorders or pain, the medical compounds being studied appear to have long term effects after a brief treatment.
“There’s a very long lasting effect, which is not seen with these other drugs you have to take every day,” said Kay Monroe, Ceruvia Lifesciences COO. “Psychedelic medicines demand to be looked at, because their mechanism of action is very different from traditional drugs, and because you don’t have to take them every day. Side effects are reduced if you don’t have to take a drug daily.”
Research suggests this benefit could be a result of how psychedelics transform the brain. Psychedelics promote synaptic growth and result in “rewiring” the brain producing long-lasting results. This appears to be the case even for compounds like BOL-148, which is chemically similar to LSD but produces no hallucinations.
In a pilot study by Harvard Medical School and Hannover Medical School, 100 percent of the patients reported significant relief from cluster headaches after receiving 3 doses of BOL-148, even when tracked over 16 weeks. Cluster headaches are typically described by sufferers as being more painful than childbirth or gunshot wounds.
Ceruvia Lifesciences owns a patent license from Harvard on BOL-148 to treat cluster headache. Their formulation is called “NYPRG-101.” BOL-148 was originally developed as a placebo and has been safely administered pre-clinically and clinically to approximately 300 people.
The company is also moving new treatments for alcoholism and opioid addiction forward with an experienced group of leading institutions, innovative collaborators, partners, academia and industry experts.
The Migraine Research Foundation says 39 million in the US suffer from migraines and 1 billion worldwide.
“Ceruvia Lifesciences is pursuing FDA approval to treat headache disorders, OCD, opioid addiction and alcoholism because the current treatments do not provide adequate results,” Turnbull said. “The large populations suffering from those conditions need better solutions.”
Ceruvia Lifesciences researches, develops, and manufactures medicines based on psychedelic research to address challenging hard-to-treat disorders, such as headache disorders and OCD. More information is available at https://ceruvialifesciences.com.
Contact Info:
Name: Carey Turnbull
Email: Send Email
Organization: Ceruvia Lifesciences, LLC
Address: 15 East Putnam Avenue, Suite #413 Greenwich, CT 06830
Website: https://ceruvialifesciences.com
Source: MarketersMedia
Release ID: 89040284