Heat Biologics: Immunotherapy Vaccine to Transform Cancer Treatment
NEW YORK, NY / ACCESSWIRE / July 16, 2015 / We all
know that cancer affects many of our friends and family, but why don’t we all
get cancer? We now know that our immune
system is continuously fighting and destroying potential cancer cells.What if we could stimulate the immune system
in cancer patients and kill the tumor? This is exactly what Heat Biologics (NASDAQ: HTBX ) is doing.
Cancer is
a devastating disease and the harsh effects of current cancer treatments are
well-known to us all.A new way of
treating cancer known as immunotherapy is transforming cancer, not in the next
5 or 10 years, but today!And Heat
Biologics is playing a significant role in this new and exciting
landscape.Very simply put their drug
activates a patient’s cancer killing T-cells (immune cells) that seek and
destroy their tumor.
The way
cancer has been treated is essentially barbaric. Patients are injected with poisons known as
chemo that kill all cells, but kill cancer cells faster. Patients are bombarded with lethal radiation,
or useful organs infested with cancer are removed, leaving patients
incapacitated for the rest of their lives.
Driven by
its proprietary platform, ImPACT, Heat is developing multiple vaccines for a
number of different cancers. Vaccines have transformed polio, tetanus, chicken
pox and measles from being deadly diseases, to merely something we take a shot
for today. Cancer could become the next disease to be virtually eliminated by a
vaccine.
ImPACT
genetically modifies human cancer cells to pump out antigens (biological agents
that target the immune system) and proteins to create an immune response and
attack a specific cancer. Not like competitor’s drugs that take days, if not
weeks, to work, Heat’s therapy takes only a few minutes. Then the vaccine lives
at the site of the tumor, continuously targeting and destroying the
cancer.
Heat’s
vaccine platform is easy to make, stays on the shelf until needed, and can be
given to anybody, regardless of their blood type or DNA makeup, which is unique
in the cancer immunotherapy market. ImPACT is far superior to immunotherapies
offered by the global behemoths I will describe below, that need to use the
patient’s own tissue to make their products work – a costly and inconvenient
method.
Currently, Merck & Co. (MRK) is in line at the FDA with Keytruda,
first approved for skin cancer (with first
quarter sales of $83 million) hoping for approval in non-small cell lung cancer
(NSCLC) where analysts predict revenues upwards of $5 billion. Not to be shut
out by its giant competitor, Bristol-Myers Squibb Co.’s (BMY) Opdivo also began
on the market for skin cancer and was approved in March for NSCLC; after
only three months Opdivo generated sales of $40 million. Both
have severe side effects which I will explain below.
Joining
the ranks of these larger companies, Heat has an ongoing Phase II trial for its
HS-110 vaccine in the treatment of NSCLC. Heat is well positioned to compete for this
market expected
to be $7 billion globally by 2019, with a high annual growth rate.
In my recent
interview with Heat founder and CEO Jeffrey Wolf, I learned that instead of
using chemotherapy that destroys all human cells, ImPACT will direct its drugs
to kill cancer cells faster with immunotherapy that activates, stimulates, and
strengthens a patient’s immune system to attack a deadly disease with no known
cure. Besides lung cancer, Heat targets bladder cancer with HS-410, now in
Phase II at 16 US clinical sites (including Johns Hopkins, known for its
extensive urological work) to potentially enter a market estimated
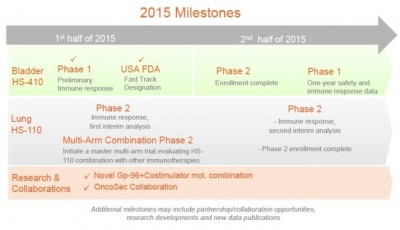
at $300 million in the next two years. Expected completion of enrollment for the bladder cancer trial is the
third quarter of next year.
One problem I discussed with Mr. Wolf were side effects of the new anti-cancer
wonder drugs that have the potential of pneumonia, holes in intestines,
hepatitis and kidney failure. Newer companies like Kite Pharma (KITE) and Juno
Therapeutics (JUNO), two much-watched darlings of the cancer immunotherapy
world, trade at a combined valuation of $7.5 billion. Both target blood cancers
but Heat is testing patients much later than Juno for lung cancer. Juno and
Kite have shown in trials to lead to an overload of the immune system, known as
a deadly cytokine storm; high fever; and serious drops in blood pressure. Medical sources claim cancer immunotherapy drugs like those of Merck and
Bristol-Myers work in only 40% of patients and we will see how they fare in
lung cancer.
The
grandfather of cancer immunotherapy, Dendreon Corp’s (DNDN), drug Provenge
sales were limited
due to the cost to the patient which was approximately $93,000, leading the
company to declare bankruptcy. The complexity of customizing the drug for each
patient was too expensive.This will not
happen to Heat. Heat’s drug is an off the shelf product, so the cost per
patient is approximately $200 for a far superior product, enabling Heat to
generate pharmaceutical grade margins, according to Wolf.
Another major coming on board to
battle in the cancer immunotherapy space for NSCLC dollars is Eli Lilly &
Co. (LLY), but the FDA is wary. Recently, the agency met with Lilly to raise concerns
about deadly blood clots. Early data showed an extension of life slightly below
Merck’s or Bristol-Meyers – a median survival of just under one year. The drug,
if approved, is expected to make around $600 million per year but with almost
10% of patients developing what could turn into a heart attack, I’m sure the
FDA will be extra cautious.
Regardless, all sales projections
discussed above make for a lucrative story for Heat.
Heat’s NSCLC trials show excellent
safety profiles with good efficacy. HS-110 activated a strong immune response
in 70% of patients, including even those far along in their disease, leading to
a substantial increase in survival – up to 300% over chemotherapy. The Phase II
trial should finish enrolling patients in Q3 2015 and data is expected to be
reported later in 2016.
Despite adverse effects from Big (and smaller) Pharma
cancer immunotherapy drugs, the industry is projected to generate $35
billion in sales over the next ten years and used to treat 60% of cancers,
according to analysts, who believe immunotherapy will cause a tectonic shift in
how oncologists view cancer therapy.
Experts believe cancer immunotherapy will
make current anti-cancer methods primitive (some say barbaric) as we do away
with flooding the body with toxic chemotherapy and removing vital organs
through surgery.
Primitive is the word Dr.
Mark Schoenberg, key opinion leader to Heat also
serving on its clinical advisory board uses to describe current methods of
treating bladder cancer (which affects over 500,000 Americans and is one of the
most expensive
cancers to treat). Standard of care is to the attack disease through surgery,
not an optimal choice for the patient because bladder tissue does not grow
back. I learned from him there are no new drugs for bladder cancer, with most
doctors using BCG – a brutal tuberculosis vaccine originating more than 40
years ago. Male patients, as most are, need a catheter inserted directly into
the penis with unpleasant effects like pus formation and skin peeling.
Foremost immunologists in the world,
Dr. Eckhard Podack, is ImPACT’s inventor and serves as chairman of Heat’s
scientific advisory board. His work is in virtually every immunology textbook;
he has a track record of developing drugs that have gained FDA approval.For example, Seattle Genetics, Inc.’s (SGEN)
main drug Adcetris for lymphoma, has driven Seattle’s $6 billion market cap.
This came out of Dr. Podack’s lab.
There are several news-related
milestones for Heat coming up in the second half of 2015. I look forward to Phase II data in
NSCLC, because many eyes on Wall Street are focused on this indication.
Heat is a promising therapy, and in
line with industry trends, where larger drug companies invest a lot of money in
cancer immunotherapy. Celgene Corp. (CELG) just spent
$1 billion in a licensing deal with Juno that extends for 10 years, with a possible
30% ownership of Juno’s stock, what analysts are calling the largest
biotechnology deal ever.After the
announcement, Juno’s shares popped 66%, underscoring the love investors have
for immunotherapy.
Immunotherapy
can be the answer to bring cancer treatment into the modern age – from a deadly
disease into one open for long-term survival, like what pharma companies did
for diabetes. Allied Market Research projects
the global cancer drug market to reach $111.9
billion by 2020, with immunotherapy and biological drugs the main driver of
growth. This is what’s driving cancer immunotherapy drug companies’ high
values.
As of March 31, Heat had
approximately $21.1 million in cash, cash equivalents, and short-term
investments, providing a runway to take it through the first quarter of 2016.
With a market cap of $53 million, recent trading at a per share price of
between $6 to $7, Heat has an unjustifiably low valuation and I believe it is
poised to at least double in the short term. I’m not alone in this prognosis,
as the two firms covering the stock have price targets of $13 and $22 per
share.
Heat is a home-run from an
efficacy/safety and economic point of view.The stock offers a
phenomenal find for those looking for a lot of upside in cancer immunotherapy,
which is now characterized by companies with billion dollar market caps.
RAY
DIRKS suggests that Readers/Investors place no more than 1% of the funds they
devote to common stocks in any one issue. It’s best to diversify.
Contact:
Jackie Rodriguez
jackie@jvprny.com
SOURCE: RAY DIRKS Research
ReleaseID: 430612