Innovus Pharmaceuticals 2014 Revenues Increase to over $1Million in Its First Full Year of Commercial Operations
Company Signs Approximately $2 Million in Minimum Annual Orders from Its Seven Commercial Partners for Its Products
SAN DIEGO, CA / ACCESSWIRE / April 1, 2015 / Innovus Pharmaceuticals, Inc. (OTCQB:INNV) (“Innovus Pharma” or the “Company”), a company focusing on the commercialization of over-the-counter (“OTC”) and consumer products for men’s and women’s health, vitality and respiratory diseases announced today that its 2014 financials have been filed on its Form 10-K Annual Report with the Securities and Exchange Commission (“SEC”) and provided a commercial and corporate update. The detailed Form 10-K is available on the SEC’s website at www.sec.gov.
Select Financial Results
Revenue
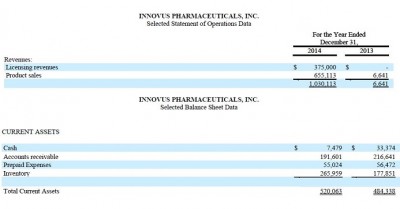
Innovus Pharma’s revenues increased to over $1,000,000 in 2014 up from $6,641 in 2013. In addition, the Company signed commercial distribution agreements with approximately $2,000,000 in minimum annual orders for its five commercial products from its seven partners around the world.
Cash Available
At December 31, 2014, the Company had over $1,000,000 million in cash available under its Line of Credit (“LOC”) with its Chief Executive Officer and President, Dr. Bassam Damaj and $191,600 in accounts receivable and no going concern opinion from its auditors.
The Company expects that its existing capital resources, revenues from sales of its products, upcoming sales milestone payments from the commercial partners signed for its products, along with the approximately $1.0 million in funds currently available for use under the LOC will be sufficient to allow the Company to sustain its operations through March 31, 2016.
Recent Business and Commercial Updates
1. Signed commercial distribution agreements with over $2 million minimum annual orders in 2015 for its five commercial products, Zestra(R), EjectDelay(R), Zestra Glide(R), Sensum+(R) and Vesele(R) from its seven commercial partners;
2. Over $410 million in potential sales milestones plus royalties in commercial partnerships (from the future efforts of its commercial partners) signed for up to 28 countries with Sothema Laboratories, Inc., Tramorgan Limited, DanaLife, Orimed/Jamp Pharma (OTC Division), Ovation Pharma and Tabuk Pharmaceuticals for the Company’s products;
3. In-licensed the product, Androferti(R) (in the U.S. and Canada) to support overall male reproductive health and sperm quality;
4. Added several large online retailers for our products, including Swanson Health, Pharmapac and Quest;
5. Acquired FlutiCare(TM) (Fluticasone Propionate Nasal Spray for Allergic Rhinitis), which we expect to launch in 2016, subject to the U.S. Food and Drug Administration’s approval of the abbreviated new drug application for the OTC version. More than 40 million units of FlutiCare(TM) nasal spray product form were sold in the U.S. in 2014, and the worldwide market is estimated to be over $1 billion annually.
“Our commercial strategy is working very well and our revenues are continuously increasing as we continue to partner our products throughout the world to reach our goal of partnering our products in 40 countries by the end of 2015,” said Dr. Bassam Damaj President & CEO of Innovus Pharma.” continued Dr. Damaj.
About Zestra(R) and FSI/AD
Zestra(R) is a patented blend of natural oils clinically-proven in double-blind placebo-controlled clinical trials in 276 women to increase in a statistical significant manner the arousal, desire and sexual satisfaction in FSI/AD women. Zestra(R) is the first NHP product to receive approval for the indication of FSI/AD in Canada as an NHP. To date, to the Company’s knowledge, no product has been approved to treat FSI/AD, a persistent or recurring inability to attain or maintain adequate sexual excitement until the completion of a sexual activity. The diagnosis can also refer to an inadequate lubrication-swelling response normally present during arousal and sexual activity causing personal distress. Published papers on the FSI/AD market size estimate it to be equal or larger than the market for erectile dysfunction in males, and possibly larger. Zestra(R) is currently available in the United States, Canada and Morocco. For more information visit www.zestra.com.
About EjectDelay(R) and Premature Ejaculation
EjectDelay(R) is an over-the-counter (“OTC”) U.S. Food and Drug Administration and Health Canada compliant proprietary topical treatment containing the drug benzocaine and indicated for treatment of premature ejaculation. The drug typically works within minutes of application to the glans of the penis. In clinical trials, the application of benzocaine has been shown to delay premature ejaculation by several minutes. For more information visit www.ejectdelay.com.
Premature ejaculation (“PE”) is the most common sexual dysfunction reported by men but is still under-diagnosed and under-treated. PE can happen at any age and its prevalence is consistent across all ages. In an article in The Journal of Sexual Medicine in 2007 Sex Med 2007, D.L. Patrick, D. Rowland and M. Rothman stated, “Global studies consistently report that 20-30% of men experience PE worldwide. This means that PE is experienced at similar rates across the globe.”
About Sensum+(R) and Reduced Penile Sensitivity
Sensum+ is a blend of essential oils and natural botanicals including rose oil, sweet almond oil, cinnamon bark oil, and other extracts. The main ingredient of Sensum+ (cinnamon oil) works by activating the Transient Receptor Potential A1 (TRPA-1) channels responsible for the heat and cold sensation of the skin and results in an increase of sensation that current users welcome and appreciate. The safety and efficacy of Sensum+ was evaluated in 2 post marketing survey studies in circumcised and non-circumcised men. A total of 382 men used Sensum+ twice daily for fourteen consecutive days followed by once daily for 8 weeks and as needed thereafter.
For more information visit www.sensumplus.com.
Reduced Penile Sensitivity (“RPS”) results from a gradual loss of penile sensitivity over time. As a person ages, the dulling effect can increase. RPS can happen at any age and its prevalence is consistent across all ages. RPS results from many causes, including diabetes, ilioinguinal nerve entrapment resulting in 20% of all hernia surgeries a year, circumcision, multiple sclerosis patients, and 2% of patients using anti-depressants drugs.
About Vesele(R)
Vesele(R) is a proprietary, novel oral dietary supplement to maximize nitric oxide’s beneficial effects on sexual function and brain health. Vesele(R) contains a patented formulation of L-Arginine and L-Citrulline, in combination with the natural absorption enhancer Bioperine(R).
The beneficial effects of Vesele(R) on sexual and cognitive functions were confirmed in a four- month US clinical survey study involving 152 patients (69 men and 83 women). Results from the clinical survey have indicated (1) improvement of erectile hardness and maintenance in men and increased sexual intercourse frequency with their partners and (2) lubrication in women, when taken separately by each. Positive effects on brain health were translated by an increase in recall of words and names.
For more information visit www.myvesele.com
About Innovus Pharmaceuticals, Inc.
Headquartered in San Diego, Innovus Pharma is focusing on the commercialization of OTC and consumer products for men’s and women’s health, vitality and respiratory diseases. The Company generates revenues from its five commercial products on a worldwide basis including Zestra(R) for female arousal, EjectDelay(R) for premature ejaculation, Sensum+(R) (for sales outside the U.S. only), Zestra Glide(R) and Vesele(R) for increasing blood flow. The Company sells its products in the U.S. online and through larger retailers such as Walmart and has seven commercial partners in 28 countries.
For more information, go to www.innovuspharma.com, www.zestra.com, www.ejectdelay.com, www.sensumplus.com, www.myvesele.com and www.myandroferti.com.
Innovus Pharma’s Forward-Looking Safe Harbor
Statements under the Private Securities Litigation Reform Act, as amended: with the exception of the historical information contained in this release, the matters described herein contain forward-looking statements that involve risks and uncertainties that may individually or mutually impact the matters herein described for a variety of reasons that are outside the control of the Company, including, but not limited to, receiving patent protection for any of its products, receiving approval or to be compliant with the requirements of any relevant regulatory authority relating to such products such as Zestra(R), to successfully commercialize such products, and to achieve its other development, commercialization, financial and staffing objectives. Readers are cautioned not to place undue reliance on these forward-looking statements as actual results could differ materially from the forward-looking statements contained herein. Readers are urged to read the risk factors set forth in the Company’s most recent annual report on Form 10-K, subsequent quarterly reports filed on Form 10-Q and other filings made with the SEC. Copies of these reports are available from the SEC’s website or without charge from the Company.
Innovus Pharma Contact:
Kevin Holmes
Chesapeake Group
info@chesapeakegp.com
T: 410-825-3930
SOURCE: Innovus Pharmaceuticals, Inc.
ReleaseID: 427408