Strong Data on Rexahn’s Non-Toxic Targeted Therapy Revealed at Top Cancer Meetings
NEW YORK, NY / ACCESSWIRE / May 28, 2015 / Rexahn Pharmaceuticals, Inc. (NYSE: RNN) took a big step toward greater US and international attention for its novel drugs in development when it presented new data on Supinoxin (RX-5902) and RX-3117 at the widely-attended 2015 American Association for Cancer Research (AACR) last month in Philadelphia, a premier venue for oncologists to see what’s new. Later this month, the company will present at the American Society of Clinical Oncology (ASCO), a scientific conference heavily attended by analysts and investors looking for the next best thing.
Conferences of this nature are known as top forums for revealing clinical results. As a poster presenter, Rexahn has a first-rate opportunity to display data to oncology leaders at not one, but two back-to-back cancer meetings, certain to garner well-deserved attention for its science. Results of clinical studies often appear in poster sessions and in abstracts (snapshots of bench or animal trials) where attendees roam large rooms to view posters and have the invaluable occasion to discuss results with researchers, one-on-one.
Rexahn’s posters covered the latest data on Supinoxin, its oral compound that inhibits P-p68 RNA helicase, or enzymes working toward a genetic effect. Supinoxin showed in earlier trials the ability to stop excessive cell growth that leads to shrinkage in tumors of the colon, lungs and ovaries, among others, with an added benefit of tackling drug-resistant cancer. Data on RX-3117, Rexahn’s potential oral treatment acting on DNA synthesis to disrupt the metabolism of cancer through apoptosis, or programmed cell death, was also displayed.
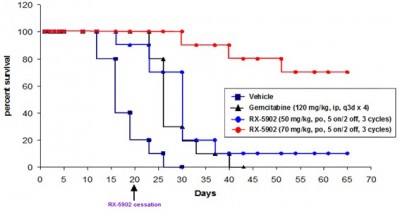
In preclinical animal studies, Supinoxin had shown strong anti-tumor properties and significant survival, with no changes in body weight of the subjects and no toxicity due to targeting tumor cells, a much better bet than current cancer therapies (see below).
Supinoxin’s (RX-5902) poster “Targeting localization and function of the RNA helicase DDX5/p68 with 1-(3,5-dimethoxyphenyl)-4-[(6-fluoro-2-methoxyquinoxalin-3-yl) aminocarbonyl] piperazine (RX-5902),” despite its daunting title, demonstrated that certain cancer cell lines can be prevented from metastasis by administration of RX-5902. The compound thus has potential for development of anti-cancer therapies.
Poster data also suggests RX-5902 may prevent metastasis in breast cancer, a global market projected to reach $24 billion by next year which I will explore later in this article.
Caption: Treatment with Supinoxin for pancreatic cancer induced in mice produced a survival benefit beyond 65 days
Source: Rexahn Pharmaceuticals
Importantly, it had been shown by Liuqing Yang, et al. as early as 2005 that p68 phosphorylation (a biochemical process that plays a big role in cell activity) is associated with abnormal cell proliferation and cancer development. Further, the close correlation between p68 phosphorylation and cancer may provide a useful diagnostic marker. Rexahn’s work so far replicates this research and as scientists know, laboratory replication is key to success in the development of drugs.
The second poster, “Fluorocyclopentenylcytosine (RX-3117) is activated by uridine-cytidine kinase 2, a potential biomarker,” supports RX-3117, a small molecule acting on DNA synthesis to disrupt the metabolism of cancer, as a biomarker through the activation of UCK2, an enzyme that gives RX-3117 power to exert its inhibition of cancer cell proliferation. In tumors expressing a high level of UCK, RX-3117 works best; in other words, the study demonstrated that RX-3117 is activated by UCK2, which can be detected by a specific antibody, lending RX-3117 its high antitumor activity by targeting tumors with a lot of UCK2 expression. Thus, RX-3117’s cancer cell specific mechanism of action was confirmed, and by virtue of its attraction to UCK2 and use as a biomarker, patients can be better selected for Rexahn’s future clinical studies, saving time and money, the true goal of targeted therapy. RX-3117 has also shown to be non-toxic in these and past studies.
In preclinical animal models, RX-3117 showed antitumor effects in colon, pancreas, kidney, ovarian, and non-small cell lung cancer, among others, with data pointing strongly to efficacy in human cancer cells resistant to popular chemotherapy gemcitabine, or Gemzar, made by Eli Lilly & Co. (LLY). I have written on this subject in the past, exploring in particular Gemzar’s treatment failure.
Supinoxin is in a Phase I dose-escalating trial to determine safety, tolerability and maximum dose for efficacy in patients with solid tumors. RX-3117 is also in a Phase I trial for solid tumors under similar study design; clinical data for both is expected in the first half of 2015 after which the best druggable targets can be chosen, assuming the studies produce good results, allowing both compounds to enter Phase II.

At the AACR, Rexahn’s poster on Supinoxin, as mentioned above, showed a drop in the migration of human triple negative breast cancer cells in a preclinical model of cancer cell metastases. According to BREASTCANCER.ORG, triple negative breast cancer tends to be more aggressive than other breast cancers, more likely to metastasize, and more likely to recur after treatment. Hormonal therapies like tamoxifen, originally discovered by AstraZeneca PLC (AZN) and Herceptin, made by Genentech, Inc., a subsidiary of Roche Holding AG (RHHBY) do not help triple negative breast cancer and come with side effects. Five-year survival rates are low. Triple negatives tend to be of a higher grade, more dangerous than other types of breast cancer. With such a strong unmet medical need, Supinoxin could provide Rexahn a shorter pathway through the FDA.
There are farther-reaching implications of Supinoxin’s effect in preventing the migration of such cells: because it acts like a prevention of metastases, Supinoxin could be effective in treatment of other malignant tumors. A recent meeting with Dr. Peter Suzdak, Rexahn’s CEO, confirms this.
As mentioned above, Rexahn will present its posters and abstracts at ASCO later this month where I suspect, given the conference’s notoriety and scrutiny by investors and analysts for company news, an influx of interest in Supinoxin and RX-3117 will follow.
In its first quarter ended March 31, 2015 Rexahn had a cash position of $29.4 million; $700,000 was received from stock option exercise. There is no debt. Research and development expenses amounted to $2.9 million versus $1.3 million in the comparable quarter last year, not surprising as Phased clinical trials ramp up, particularly for advancement of Archexin, Rexahn’s compound for kidney cancer now in Phase II. General and administrative costs were $1.5 million, on par with last year’s figure showing good handling of internal spending. Net loss improved from ($0.09) in 3Q14 to ($0.02) in this year’s quarter, both figures including the fluctuating nature of accounting for stock price fair value adjustments.
As a clinical stage biopharmaceutical company, risks to investing in Rexahn are high. Market capitalization is $134 million, considered small. Revenues may not materialize for a number of years; regulatory issues may cost the company additional money; a capital infusion may become critical. Competition for novel solid tumor remedies is fierce. A certain, clear risk with small-cap stocks is illiquidity and lack of news flow, but Rexahn has a healthy average three-month volume of over 500,000 shares traded and news from the company has been flowing well.
I expect excitement surrounding this new data for Supinoxin and RX-3117 to create industry buzz, contagious to Wall Street. Rexahn’s participation at large cancer conferences, typically lasting five days with plenty time for networking, signals to me progress in their clinical and scientific stature, and with trials progressing nicely, a greater opportunity to enjoy entrance into the global oncology drug market estimated to reach $111.9 by 2020, not counting growth of new medicines as key cancer drugs Erbitux, Rituxan and Avastin go off patent.
Every oncologist’s dream is a cancer therapy that is non-toxic, tumor targeted, with the potential for high efficacy, bringing them closer to their goal of personalized medicine to treat very sick patients. Rexahn’s new data, backed by strong science and revealed in two high-profile settings puts them on the frontline of cancer exploration, practically ensuring attention from clinicians and investors alike. Valuation of shares do not reflect potential, and investors willing to trade risk for reward, I believe, won’t be disappointed.
Media contact for Small Cap Forecasting, Inc.
Jackie Rodriguez/JVPR NY
jackie@jvprny.com
SOURCE: Small Cap Forecasting, Inc.
ReleaseID: 429322