Vycor Medical Releases Financial Results for the Twelve Months Ended December 31st, 2014
Vycor VBAS up 23% compared to 2013; Non-GAAP Net Loss reduced by 7%
BOCA RATON, FL / ACCESSWIRE / March 31, 2015 / Vycor Medical, Inc. (“Vycor”) (OTCQB:VYCO), today announced financial results for the year ended December 31, 2014.
Company Highlights
Vycor VBAS
– ViewSite Brain Access System (VBAS) has continued to gain traction through product approval in 35 new U.S. hospitals during the year, and is now approved in more than 180 hospitals in the U.S. with additional hospitals in the approval process.
– Internationally, Vycor Medical entered into evaluation or distribution agreements during the year covering Brazil, France, India, Israel, Russia, South Africa, Sri Lanka and Taiwan.
– Scientific data on VBAS’ clinical advantages continues to be built: surgeons from Weill Cornell Medical College presented their work on minimally invasive neurosurgical approaches using VBAS at neurological conferences in Xian, China, Prague and the CNS in Boston, and had two peer review papers detailing this work published in March 2015, including one in the prestigious Journal of Neurosurgery which featured VBAS on its front cover; and surgeons from Sapporo Medical University in Japan also published a study using VBAS. Peer reviewed clinical data is very important in driving surgeon and hospital adoption which in turn drives revenues.
– Entered into a manufacturing agreement with an Asian-based manufacturer for the production of two new smaller VBAS devices that will facilitate endoscopic work within the ventricles including the placement of catheters. Vycor is progressing the manufacturing build and validation program for these devices which will be available in the fourth quarter of 2015.
– Completed the development of a new VBAS prototypes specifically designed to house the optical pointers utilized in image-guided systems. The optical pointers will be firmly held in place in the introducer through a novel approach while the working channel will remain unchanged. The Company plans on having four new IGS compatible devices, available in the second half of 2016.
NovaVision
– Developed and soft-launched NeuroEyeCoach(TM) in the U.S. the first commercially available saccadic therapy internet-delivered to patients’ computers in their own homes. The program re-trains the ability of a patient to scan the environment, re-integrate left and right vision and make the most of their remaining visual field and is highly complementary to VRT.
– Developed and now launching two Professional versions of NeuroEyeCoach(TM) for rehab centers or other clinics to treat patients while in their care, and for physicians.
– Completed development in March 2015 of VRT, re-engineering the therapy to be internet-delivered and streamlining business processes to enable a significant reduction in cost to the patient. This will be commercially launched during the second quarter.
– Received approval for and entered into agreement to offer its VIDIT diagnostic device throughout the 100+ network of HealthSouth rehabilitation centers in the U.S., further validating the NovaVision’s technology and therapies.
Peter Zachariou, Chief Executive Officer of Vycor, commented, “Vycor VBAS continues to build value and momentum and we are executing on our new product development plan for our new small VBAS units and new IGS-compatible devices. We have announced the completion of the NovaVision development strategy, and are gearing up for marketing the truly scalable and most affordable, comprehensive therapy suite for those suffering visual disorders from neurological damage such as stroke. With the $5 million offering completed in the second quarter and the $2.4 million debt exchange by our largest shareholder in the third quarter, we continue to build shareholder value through the execution of our previously articulated strategy.”
Financial Results
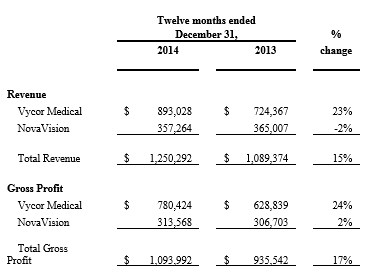
For the year ended December 31, 2014, the Company reported revenue of $1,250,292, a 15% increase over 2013. The Vycor Medical division (VBAS) generated revenue of $893,028, a 23% increase compared to the same period in 2013. Gross profit for 2014 was $1,093,992, a 17% increase over 2013, an increased margin of 87% versus 86% in 2013.
For the twelve months to December 31, 2014 the Company reported non-GAAP Operating Expenses of $2.6 million compared to $2.5 million in the same period in 2014. The non-GAAP net operating loss was $1.5 million compared to $1.6 million in 2014, a reduction of 4%, and the non-GAAP net loss was $1.7 million , a reduction of 7%. The increase in expenses is as a result of Vycor’s development work and gearing up for marketing of new products in 2015.
Cash and cash equivalents were $1.9 million at December 31, 2014 and Shareholders’ Equity was $2.9 million
Reconciliation of Non-GAAP Information and Pro Forma Balance Sheet
Non-GAAP Reconciliation
Management uses certain non-GAAP financial measures (including non-GAAP operating expenses and non-GAAP net loss and loss per share), which exclude non-cash amortization of acquired intangible assets, non-cash stock-based, one-time Offering costs and the change in value of derivative warrant liability. Management does not consider these costs in evaluating the continuing operations of the Company. Therefore, management calculates the non-GAAP financial measures provided in this earnings release excluding these costs and uses these non-GAAP financial measures to enable it to analyze further, and more consistently, the period-to-period financial performance of its core business operations. Management believes that providing investors with these non-GAAP measures gives them additional important information to enable them to assess, in the same way management assesses, the Company’s current and future continuing operations. There are limitations in using these non-GAAP financial measures because they are not prepared in accordance with GAAP and may be different from non-GAAP financial measures used by other companies. These non-GAAP financial measures should not be considered in isolation or as a substitute for GAAP financial measures. Investors and potential investors should consider non-GAAP financial measures only in conjunction with the Company’s consolidated financial statements prepared in accordance with GAAP. Set forth below are reconciliations of the non-GAAP financial measures to the comparable GAAP financial measures.
On a GAAP basis the Company reported Operating Expenses of $3.8 million, a net operating loss of $2.7 million, a net loss of $4.0 million and a loss of $0.54 per share.
Vycor’s GAAP operating costs for year ended December 31, 2014 include non-cash amortization of acquired intangible assets ($234,467), non-cash stock compensation charges ($376,662), and one-time offering costs ($581,702). Vycor’s other income includes change in the value of warrant derivative liability (loss of $252,633), loss on extinguishment of debt ($682,039), loss on extension of warrants ($146,488) and loss on foreign currency exchange ($105,685). The Company is providing additional non-GAAP financial measures that exclude these charges and expenses, and reconciliation of GAAP to non-GAAP results is provided in the tables included in this release.
On a non-GAAP basis, taking into account these adjustments, Operating Expenses for the year ended December 31, 2014 were $2,633,309, non-GAAP net operating loss was $1,539,317, and non-GAAP loss was $1,670,036 or $0.22 per share.



(1) Non-Cash Amortization on acquired intangible assets. These are non-cash charges related to acquired intangible assets such patents and software which can be impacted by the timing and magnitude of acquisitions. We consider our operating results without these charges when evaluating our ongoing costs and performance, and therefore exclude such charges when presenting non-GAAP financial measures.
(2) Non-Cash Stock-based compensation expense consists of expense relating to stock-based awards issued to employees, outside directors and non employees including stock options, restricted common stock, and warrants. Because of varying available valuation methodologies, subjective assumptions and the fact that these amounts vary in size and timing, we believe that the exclusion of stock-based compensation expense allows for a more accurate comparison of our financial results to previous periods. In addition, we believe it is useful to investors to understand the specific impact of stock-based compensation expenses on our operating results.
(3) Offering Costs comprises the broker commissions, banking fees, legal fees and other costs associated with the five separate closings of an offering of units of Common Stock and Warrants (the “Offering”) during the the period January to April, 2014. These costs are one-time for the period of the Offering and are disregarded by management in evaluating and predicting earnings trends and are therefore excluded by us when presenting non-GAAP financial measures.
(4) Loss on Extinguishment of Debt. In August, 2014, the Company entered into a series of agreements with Fountainhead, along with certain other related and non-related parties (together, the “Fountainhead Parties”), to exchange all of the parties’ $2,355,587 of debt into Company preferred equity of equivalent value. Under Applicable Accounting Guidance ASC 405 and 470, the exchange is accounted for as an extinguishment of debt. The Company is required to compare the carrying value of the securities being extinguished (without placing any value on security preference, default protection, conversion rights or other matters) with the fair value of the securities being issued in exchange. The fair values were determined using a variety of techniques including Black-Scholes, taking into account stock volatility but not, importantly, stock illiquidity, to derive a theoretical fair value. The securities issued in exchange are then recorded on the balance sheet at this fair value and the difference between fair value of the new securities and the carrying value of the extinguished securities is recognized in the income statement as a gain or loss. Because this was a one-time transaction and is calculated using subjective valuation assumptions, we believe that the exclusion of the loss on extinguishment of debt allows for a more accurate reflection of our financial result, and has therefore been excluded by us when presenting non-GAAP financial measures.
(5) Loss on Extension of Warrants. In August, 2014, in a transaction unrelated to the exchange in Note 4, Fountainhead entered into an agreement with the Company preventing it from selling any Vycor Shares currently held by Fountainhead below $4.50. In return, the Company agreed to extend the life of certain of Fountainhead’s existing warrants expiring in 2015 to the same 3-year term as the warrants being issued under the exchange. The fair value of the extended terms were compared to the fair value of the existing terms, using Black-Scholes. The extended warrants were recorded on the balance sheet at this fair value and the difference between fair value of the extended terms and of the existing terms was recognized in the income statement as loss. Because this treatment is limited in time and is calculated using subjective valuation assumptions, we believe that the exclusion of the derivative liability on the balance sheet, and the change in valuation on the statement of operations, allows for a more accurate reflection of our financial result, and has therefore been excluded by us when presenting non-GAAP financial measures.
(6) Derivative Liability: Warrant. The Company accounts for the remaining 34,723 Series A Warrants issued in connection with the Offering which still carry anti-dilution rights in accordance with the guidance contained in ASC 815-40-15-7D, whereby under that provision, because they have anti-dilution rights, they do not meet the criteria for equity treatment and must be recorded as a liability. Accordingly, the Company classifies the warrant instrument as a liability at its fair value and adjusts the instrument to fair value at each reporting period. This liability is subject to re-measurement at each balance sheet date until exercised or until the anti-dilution provisions contained within the warrant agreements expire, and is classified in the balance sheet as a current liability. Because this treatment is limited in time and is calculated using subjective valuation assumptions, we believe that the exclusion of the derivative liability on the balance sheet, and the change in valuation on the statement of operations, allows for a more accurate reflection of our financial result, and has therefore been excluded by us when presenting non-GAAP financial measures.
About Vycor Medical, Inc.
With corporate headquarters in Boca Raton, FL, Vycor Medical, Inc. (“Vycor”) is a publicly traded company (OTCQB: VYCO) dedicated to providing the medical community with innovative and superior surgical and therapeutic solutions and has a growing portfolio of FDA cleared medical solutions that are changing and improving lives every day. The Company operates two business units: Vycor Medical and NovaVision, both of which adopt a minimally or non-invasive approach. Both technologies have exceptional sales growth potential, address large potential markets, have the requisite regulatory approvals and are commercialized and generating revenue.
Vycor Medical’s ViewSite(TM) Surgical Access Systems (VBAS) is a suite of clear cylindrical minimally invasive disposable devices that hold the potential for speedier, safer and more economical brain surgeries and a quicker patient discharge. VBAS is designed to optimize neurosurgical site access, reduce patient risk, accelerate recovery and add tangible value to the professional medical community. The company is ISO 13485:2003 compliant, has U.S. FDA 510(k) clearance for brain and spine surgeries and full regulatory approvals for brain in Australia, Canada, China, Europe (EU – Class III), Korea and Japan and is seeking or has partial regulatory approvals in Brazil, India, Russia, Taiwan and Vietnam. For an overview of Vycor Medical’s VBAS see VBAS Video.
NovaVision develops and provides science-driven neurostimulation therapy and other medical technologies that help improve and partially restore sight in patients with neurological vision impairments. The company’s proprietary Visual Restoration Therapy(R) (VRT) platform is clinically supported to improve lost vision resulting from stroke, traumatic brain injury (“TBI”), or other acquired brain injuries. VRT is the only FDA 510K cleared medical device in the U.S. aimed at the restoration of vision for neurologically induced vision loss and can be prescribed by any Physician. VRT also has CE Marking for the EU. NovaVision also provides Neuro Eye Therapy (NeET) in the EU, aimed at increasing visual sensitivity deep within the field defect.
The Company has also developed a therapy called NeuroEyeCoach(TM). The NeuroEyeCoach(TM) therapy is highly complementary to VRT(TM). The two therapies address different visual disabilities each of which results from neurologically-induced vision loss – a loss of visual field as well as difficulty with eye movement, affecting the ability to integrate visual information. VRT provides partial restoration of the patient’s lost visual field; NeuroEyeCoach(TM) is designed to increase the efficiency of eye movement and re-train the patients’ ability to integrate visual information between the left and right hand side. For an overview of NovaVision see NovaVision Video.
For the latest information on the company, including media and other coverage, and to learn more, please go online at www.vycormedical.com, www.vycorvbas.com or www.novavision.com.
Safe Harbor Statement
Information in this document constitute forward-looking statements or statements which may be deemed or construed to be forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. The words “forecast,” “anticipate,” “estimate,” “project,” “intend,” “expect,” “should,” “believe,” and similar expressions are intended to identify forward-looking statements. These forward-looking statements involve, and are subject to known and unknown risks, uncertainties and other factors which could cause Vycor Medical’s actual results, performance (financial or operating) or achievements to differ from the future results, performance (financial or operating) or achievements expressed or implied by such forward-looking statements. The risks, uncertainties and other factors are more fully discussed in Vycor Medical’s filings with the U.S. Securities and Exchange Commission. All forward-looking statements attributable to Vycor Medical herein are expressly qualified in their entirety by the above-mentioned cautionary statement. Vycor Medical disclaims any obligation to update forward-looking statements contained in this estimate, except as may be required by law.
Vycor Medical, Inc. Contacts:
6401 Congress Avenue
Boca Raton, FL. 33487
(561) 558-2020
info@vycormedical.com
SOURCE: Vycor Medical, Inc.
ReleaseID: 427346