Biom’up reports 2018 full-year results and confirms strong commercial uptake in 1st quarter of 2019
SAINT-PRIEST, FRANCE / ACCESSWIRE / April 30, 2019 / Biom’up (the “Company”), a specialist in surgical hemostasis, today announced its full-year results for the year ending December 31, 2018, as approved by the Company’s Board of Directors on April 29, 2019, and provided an update on its operations.
Financial Highlights:
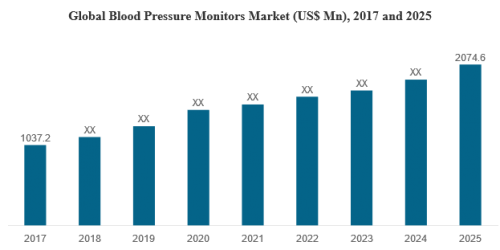
Total sales of EUR1.2 million including first sales of HEMOBLAST Bellows in the US and Europe;
Operating expenses increased to EUR38.9 million (2017: EUR18.2 million), largely due to one-time U.S. and European commercial roll-out costs and manufacturing scale-up plans executed to meet market demand;
A total of EUR51 million in a mix of equity and convertible bonds secured in 2018 to finance growth; and
Consolidated cash position as of December 31, 2018 at EUR30.6 million (EUR33.0 million at December 31, 2017).
Operational Highlights:
Strong commercial uptake of HEMOBLAST Bellows after U.S. and European roll-outs, with 25% monthly sequential order growth since launch in July 2018 through end of 1Q 2019;
As of the end of March 2019, 61% of the company’s customers have ordered more than once, a number reaching 74% in the U.S. and French markets;
Completion of the 2018 manufacturing scale-up, including its regulatory approval, with a maximum capacity of 4,000 units a month achieved as of December 31, 2018. The Company confirms its objective of 7,000 units to be reached per month in the course of 2019; and
U.S. and European regulatory approvals for HEMOBLAST Bellows Laparoscopic Applicator in January 2019 and July 2018 respectively, opening new market segments representing close to a total of one million surgeries in the U.S and Europe annually.
“2018 was a turning point in the history of Biom’up, with the global launch of our flagship product, HEMOBLAST Bellows. Efforts in 2018 were focused on two key initiatives: executing and obtaining approval for a major manufacturing production scale-up and expanding our market reach by strengthening our marketing initiatives on both sides of the Atlantic. We start 2019 on strong footing. We see growing market demand for our product as it is becoming more widely available, as evidenced in the past few months by a significant number of recurring orders coming from hospitals,” said Etienne Binant, CEO of Biom’up. “Equity markets and our existing financial partners have continued in 2018 to give us the support necessary to finance these efforts. I would like to thank them, just as I would like to thank the entire Biom’up family for the on-going commitment to delivering the best hemostatic products safely to patients worldwide.”
The French-language full year financial report for the 2018 fiscal year is being filed with the French Autorité des marchés financiers today and is available to the public. It can be downloaded from the Company’s website (www.biomup.com), folder Investisseurs / Documentation.
ENCOURAGING MARKET ADOPTION AFTER SUCCESSFUL U.S. AND EU ROLLOUTS
United States
In May 2018, the Company announced the finalization of its commercial infrastructure to support HEMOBLAST Bellows’ launch in the United States. The Company has recruited an experienced management team, now headed by George Makhoul, and independent specialist sales representatives to drive the nationwide marketing and sales campaign of HEMOBLAST Bellows. This commercial organization provides, in the Company’s opinion, sufficient geographic coverage to ensure HEMOBLAST Bellows’ commercial success.
As of December 31, 2018, 42 individual hospital sites have purchased HEMOBLAST Bellows, indicating that the product has already successfully passed multiple hospital evaluations.
Europe
As previously communicated, in Europe, the French and DACH markets are directly addressed through the Company’s own sales force. In other European markets, Biom’up is actively identifying distribution partners.
In Europe, Biom’up focused the delivery of HEMOBLAST Bellows to key customers in France and the German-speaking countries of Europe (Germany, Austria, Switzerland, also known as DACH). As of December 31, 2018, 21 hospitals in France – of which 9 are university hospitals – already use HEMOBLAST Bellows in their clinical routines, an encouraging sign of market adoption. In addition, 15 hospitals among some of the most renowned in Germany, Austria and Switzerland have placed several orders.
Other geographies
Biom’up is making substantial progress in Australia where the Company has submitted a reimbursement dossier for HEMOBLAST Bellows in the Prostheses list. This dossier comes in addition to the regulatory submission for approval of the product, initially filed on March 15th, 2018. The final decision of the Australian authorities is now expected in the second half of 2019.
In Japan and New Zealand, product registrations are now expected within the next two years.
MANUFACTURING SCALE-UP EXECUTED SUCCESSFULLY TO MEET COMMERCIAL DEMAND
The first shipment of HEMOBLAST Bellows from the Company’s French production site in Saint-Priest to the Company’s central distribution center in the U.S. in June 2018 confirmed that the supply chain is operational, reliable and fully compliant with U.S. regulations. This allowed the first sales of HEMOBLAST Bellows in the U.S. in July 2018.
In November 2018, the Company announced completion, under the supervision of Thierry Darnis (Global Vice President, Manufacturing & Engineering), of a rigorous scale-up of its French manufacturing facility to address the increasing commercial demand for HEMOBLAST Bellows globally. The scale-up, coming on top of capacity freed by the discontinuation of legacy products, included the doubling of the number of shifts and the upgrade and full validation of six key internal and outsourced processes. Taken together, these steps allowed for an increase of HEMOBLAST Bellows’ production to more than 4,000 units per month. The Company expects this number to grow to 7,000 units per month in the course of 2019 and to reach a production rate of 9,000 units per month by the end of 2019. These revised numbers are an improvement over what had been previously communicated. All updated processes were thoroughly reviewed and approved by the FDA and the Company’s notified body, BSI.
Plans to increase the Company’s manufacturing capacity to meet demand in the mid-term include, in chronological order:
increased capacity at its current site;
more extensive recourse to sub-contractors; and
building a new production facility, now expected in 2021. This new site would bring the Company’s total production capacity to 45,000 units of HEMOBLAST Bellows per month by the end of 2022.
PORTFOLIO EXPANDED WITH HEMOBLAST BELLOWS LAPAROSCOPIC APPLICATOR
In July 2018, Biom’up obtained CE-marking for its laparoscopic applicator, enabling surgeons to use HEMOBLAST Bellows hemostatic powder for both open and laparoscopic surgery, while continuing to benefit from the same product strengths of efficacy, simplicity, and on-demand use. In January 2019, the product obtained the FDA approval for its commercialization in the US, and first U.S sales took place as early as April 2019.
FINANCIAL PERFORMANCE (Key Figures)
Income statement and Cash flows
In EUR 1,000
2018
2017
Hemostatic products
627
189
Other sales
48
492
Discontinued products
500
1 086
Revenues
1 176
1 766
Operating expenses
(38 948)
(18 219)
Current operating profit/(loss)
(37 772)
(16 443)
Operating Profit/(loss)
(38 275)
(17 713)
Net financial income
(2 315)
(13 569)
Net income/(loss) of consolidated operations
(40 590)
(31 283)
Audit procedures on statutory and consolidated annual financial statements have been performed and the auditors are in the process of issuing their audit reports.
Revenue
The Company’s decision to focus resources on its lead product, HEMOBLAST Bellows, and discontinue the sale of its historical products (COVA, COVAMESH and MATRIBONE) led to a decline in revenues in 2018 compared to 2017. Sales in 2018 included EUR0,5 million (2017: EUR1,1 million) of revenues generated by the disposal of the remaining inventory of these historical products in France.
In an encouraging sign, sales of HEMOBLAST Bellows grew to EUR0,6 million, of which EUR0,3 million were in Europe and the remainder in the U.S. With the launch of the product in the second half of 2018, the Company saw a strong commercial uptake of HEMOBLAST Bellows in the market. The Company thus believes its 2019 revenues will grow strongly versus 2018.
Operating profit/(loss)
After reinforcing commercial teams and successfully launching HEMOBLAST Bellows in the U.S. and EU, operating expenses rose significantly in relation to 2017, to EUR38.9 million (EUR18.2 million in 2017). This increase in part reflects the increase in operating loss to EUR38.3 million (2017: EUR17.7 million).
In addition, the Company invested heavily in implementing an appropriate organization and infrastructure to support HEMOBLAST Bellows’ commercial uptake and ensure successful operation following rigorous scale-up of its French manufacturing facility. In consequence, staff costs are up twofold from the last fiscal year.
In 2019, the Company foresees its operating expenses decreasing significantly compared to 2018 as most of the one-time expenses of the launch are behind it and its expenditures grow in proportion to the revenue growth.
Cash flows
The consolidated cash balance at December 31, 2018 stood at EUR30.6 million compared to EUR33.0 million at December 31, 2017. Cash inflows amounted to EUR51,0 million in 2018, originating mainly from the capital increase in February 2018, the issue to Athyrium of a first tranche of bonds with stock warrants attached in March 2018, as well as the private placement financing and the issue to Athyrium of a second tranche of bonds with stock warrants attached in December 2018. Cash outflows were EUR53.6 million, including EUR8.6 million in loan repayment, EUR7.4 million in capital expenditures and EUR37.6 million in operating expenses.
The Company will continue to need important financial resources to cover its planned operating and investing activities. In light of the material uncertainty linked to finding new sources of financing and the consequences of a breach of minimum liquidity ratio under the Athyrium credit facility, the Company could face a going-concern issue in the normal course of its activities, as will be outlined in the auditors’ report to our annual financial statements.
LAYING THE FINANCIAL FOUNDATION TO EXECUTE GROWTH STRATEGY
In February 2018, Biom’up executed a capital increase of EUR16 million (including additional paid-in capital) entailing the issuance of 1,452,418 new shares by means of a public offering at a price per share of EUR11.00.
In December 2018, Biom’up performed a successful closing of a private placement of EUR7.7 million through a capital increase by the issuance of 1,597,332 new shares for the benefit of a category of beneficiaries at a price of EUR4.80 per share.
In March 2018, the Company also secured a EUR25 million bond issue with stock warrants attached subscribed by Athyrium Opportunities III Acquisition LP, a fund managed by Athyrium Capital Management, L.P. In December 2018, Biom’up exercised the second tranche of this bond financing for EUR3 million out of the EUR10 million then still available. EUR7 million remain available under conditions. The deadline to draw down the balance has been extended to December 31, 2019.
Net proceeds from the capital increases and bond issues are used to finance HEMOBLAST Bellows’ commercial ramp up in the U.S. and Europe, continuing regulatory efforts to both maintain the authorizations already obtained in Europe and the U.S. and obtain authorizations in new countries (Australia, Japan) and continuing efforts to pursue clinical developments necessary for the product’s authorization in the year ahead for new therapeutic targets including spinal surgery in the United States. It is also intended that those proceeds will be used for the construction of a second manufacturing facility to support HEMOBLAST Bellows’ deployment globally, and to fund preclinical and clinical trials for HEMOBLAST Bellows and HEMOSNOW. A portion of the proceeds from the March 2018 bond issue were also used to repay in advance the Kreos venture loan for an amount totaling EUR7.6 million (including interests and penalties).
CORPORATE
Changes in governance
On March 29, 2018, Athyrium Opportunities III Acquisition LP, acting through its initial representative, Mr. Laurent Hermouet, joined the Company’s Board of Directors as a non-voting observer (censeur).
In May 2018, Ms. Marie-Laure Pochon resigned from the board of directors.
On July 3, 2018, the Company announced the arrival of Ms. Caroline Lang as independent director and Ms. Janice Hogan as non-voting observer (censeur) to strengthen its focus on the U.S. market, following the departure of Mr. Laurent Higueret as director and Bpifrance Investment as non-voting observer.
On September 4, 2018, the Company announced Dr. Peter Byloos’ addition to the Board as an independent director.
Both new director mandates (Caroline Lang and Dr. Peter Byloos) will be submitted to shareholders’ vote at the next annual general meeting, each for a duration equal to that of the mandate of his/her predecessor.
2018 profit sharing plan
On August 27, 2018, the Board of Directors adopted the terms of the following profit-sharing agreements:
Free shares representing 1.36% of the existing share capital were granted to employees and corporate officers of the group pursuant to the authorization granted by resolution fourteen of the general meeting of August 31, 2017;
Stock options representing 2.81% of the existing share capital were granted to employees of the U.S. subsidiary of the Company, Biom’up USA, Inc., pursuant to the authorization granted by resolution eleven of the general meeting of June 5, 2018; and
Warrants representing 0.12% of the existing share capital were granted to an independent director and a service provider (Key Opinion Leader), pursuant to the delegation of authority granted by resolution twelve of the general meeting of June 5, 2018.
POST-CLOSING EVENTS AND OUTLOOK 2019
The continuation of the strategy initiated in 2018
In January 2019, Biom’up announced the renewal, for the next seven years, of its supply agreement for human-derived thrombin, a key component of HEMOBLAST Bellows, only available from a limited number of suppliers. With this new agreement, the Company ensures an adequate supply of its key component to meet its commercial goals.
Simultaneously, Biom’up announced the signature of an important exclusive agreement with LHC (Life Healthcare), a leading independent distributor of medical devices and healthcare solutions in Australia, for future distribution of its medical device pending approval for sale in Australian market. LHC and Biom’up have defined clear milestones for the market development with an initial focus on spine and cardiac surgeries.
Finally, on April 17, 2019, the FDA approved the IDE (Investigational Device Exemption) application for HEMOSNOW, a hemostatic dry powder made from porcine collagen and bovine-derived chondroitin sulfate developed by Biom’up for managing minimal and mild levels of bleeding during surgical procedures. The Company initially submitted an IDE application to the FDA for HEMOSNOW in January 2019. The Company plans to prepare the launch of the clinical testing required to obtain approval in the United States in 2020.
Focus on key initiatives
Going forward, Biom’up will continue to focus on the following strategic initiatives:
Continue expanding the sale of HEMOBLAST Bellows in the U.S. and Europe;
Expand HEMOBLAST Bellows distribution networks in geographies outside of the U.S. and Europe;
Increase HEMOBLAST Bellows production;
Develop HEMOBLAST Bellows through clinical trials to expand its scope of application, in spine and beyond, but also through post-marketing, follow-up clinical studies to further document is benefits; and
Seek approval of HEMOSNOW in the U.S. market (launch of U.S. clinical studies in 2020 in connection with a PMA application with the FDA).
While the Company expects its 2019 revenues to grow strongly versus 2018 and its operating expenses to decrease significantly over the period, it is revising to 2023 (v. 2022 initially) its objective of a 15% market share in the U.S. and main European countries and plans on announcing new objectives in the coming months to replace its initial guidance.
Contacts
Biom’up
Chief Financial Officer
Jean-Yves Quentel
investisseurs@biomup.com
+33 4 86 57 36 10
MC Services AG
Internationale Investor and Public Relations
Anne Hennecke
anne.hennecke@mc-services.eu
+49 211 529252-22
About Biom’up
Founded in 2005 and based in the Lyon suburb of Saint-Priest (France), Biom’up develops and commercializes hemostatic products based on patented biopolymers designed to simplify the surgeons practices for open and minimally invasive surgical procedures, including laparoscopic, in multiple specialties such as cardiac, general, and orthopedic surgery. The Company’s lead product, HEMOBLASTTM Bellows and its laparoscopic applicator are marketed in Europe and the United States.
Since its creation, Biom’up has benefited from the support of prominent European and U.S. investors. The Company’s shareholders include Bpifrance (including its Innobio fund), Gimv, Lundbeckfond, Athyrium Capital, Financière Arbevel and Invesco, as well as all the Company’s management team. Biom’up successfully completed its IPO on Euronext Paris, raising EUR42.5 million in October 2017.
Since then, the Company carried out a EUR16 million capital increase in February 2018 and a EUR7.7 million capital increase by means of a private placement in December 2018. It also entered into a EUR25 million bond financing agreement with Athyrium, a U.S. fund specializing in innovative companies in the healthcare sector, in March 2018, that was brought to EUR28 million in December 2018.
About HEMOBLAST
HEMOBLAST Bellows is a hemostatic product to control bleeding in a broad range of open and minimally invasive surgical procedures including laparoscopy for multiple specialties such as cardiac, general, and orthopedic surgery.
Uncontrolled bleeding is a major surgical complication associated with higher mortality, longer hospitalization and higher rates of transfusions and reoperations. Beyond its impact on patient’s health, this major complication causes excess costs in all surgical specialties and is a burden for hospital budgets across the globe. HEMOBLAST Bellows is the only surgical hemostatic agent approved by the FDA based on the validated SPOT GRADE(TM) Surface Bleeding Severity Scale (SBSS), which demonstrates the ability to control a range of bleeding from minimal (oozing), mild (pooling) and moderate (flowing) bleeding. HEMOBLAST Bellows is proven to control bleeding with flow rates up to 117 mL per minute. Due to its efficacy, versatility and ease of use, HEMOBLAST Bellows is quickly becoming a popular choice amongst U.S. surgeons looking for new options to control surgical bleeding challenges.
Biom’up obtained CE Marking for HEMOBLAST Bellows in December 2016. On the basis of compelling preliminary results (93% effectiveness at 6 minutes, compared with 74% for the control arm) in a major clinical trial, FDA approval for HEMOBLAST Bellows in December 2017, seven months ahead of the original plan. This allowed for the commercial roll-out of its lead product in the U.S. in the summer of 2018.
In July 2018, Biom’up additionally obtained CE Marking for its HEMOBLAST Bellows Laparoscopic Applicator designed to deliver the HEMOBLAST Bellows powder in all minimally-invasive procedures. In January 2019, the Company obtained the respective approval for HEMOBLAST Bellows Laparoscopic Applicator in the U.S. This has opened up new market segments, representing approximately 500,000 and 443,000 surgeries per year in Europe and the US respectively.
Currently the Company is working to expand the range of applications for HEMOBLAST Bellows. In addition, the approval from the Australian health authorities for the Company’s lead product is expected during the second half of 2019.
SOURCE: Biom’up
ReleaseID: 543477