~ Company Plans to Gain Access to the #7 Level (Sangria) Adit ~
VANCOUVER, BC / ACCESSWIRE / June 24, 2020 / VanGold Mining Corp. (the "Company" or "VanGold") (TSXV:VGLD) announces plans to begin clearing the El Pinguico shaft at its El Pinguico silver and gold project, located 7km south of the city of Guanajuato, Mexico.
Clearing the El Pinguico Shaft:
Preparations are underway to begin clearing approximately 30m of debris from the bottom of the El Pinguico shaft, one of three shafts that gave access to the historic El Pinguico mine.
The company will be attaching a one tonne capacity hoist to its previously installed steel head-frame in order to hoist material that has fallen to the bottom of the El Pinguico shaft since mining ceased in 1913. The company has begun hiring a team of experienced underground miners to perform the delicate work of removing this material safely while maintaining the structural integrity of the shaft. All activities will be performed according to Mexican regulations regarding the prevention of the spread of COVID-19.
The Company plans to begin this operation in mid-July, and has started the process of hiring key personnel for this task. It anticipates that once crews begin this work that it will require approximately three weeks to clear the shaft down to the #7, or ‘Sangria' adit level.
Permitting:
All necessary permits to operate the one tonne capacity hoist, and remove the material from the bottom of the shaft have been attained. The Company's only further regulatory requirement is to alert the local municipal government of its intention to commence this activity, which the Company intends to do in the first week of July.
VanGold Plans Three Initiatives After Clearing Shaft:
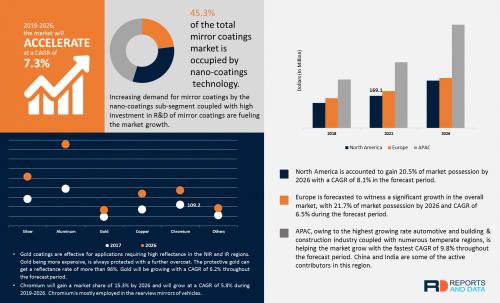
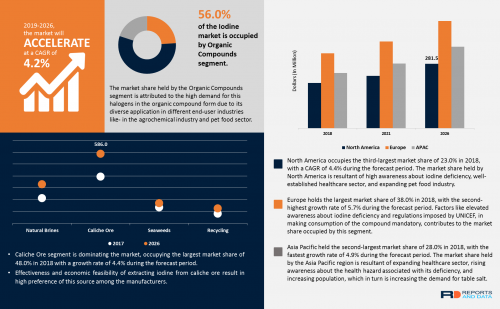
Sampling the bottom of the underground stockpile: The underground (UG) stockpile consists of material that in 2012 the Mexican Geological Survey (SGM) agency determined to be 148,966 tonnes in size.[1] In 2017, VanGold conducted a trenching program at the top of the UG stockpile. This program resulted in a weighted average of all of the trench samples of 1.75 gpt Au and 183 gpt Ag, which is similar to the grades quoted in 2012 by SGM of 1.66 gpt Au and 143 gpt Ag.[2] Once crews remove the material at the bottom of the El Pinguico shaft VanGold will be in a position to properly sample the bottom of the UG stockpile and determine whether the silver and gold grades, established by VanGold's trenching in 2017 on the top of the stockpile, extend to the bottom of the stockpile.
2. Inspecting the #7 Sangria Adit: Clearing the El Pinguico shaft will allow crews to enter and inspect the mine's #7 Adit – also known as the "Sangria" adit. This adit may provide a potential safe and inexpensive haulage way to bring the UG stockpile material to surface for onward delivery to a nearby mill for processing. The Company will study this approach for bringing the UG stockpile material out of the mine; however fully refurbishing the El Pinguico shaft is also a potential alternative. The decision on which of these possibilities the Company will pursue will be made once the Sangria adit is entered and fully inspected.
3. Sampling of the Colmillo Stope: Once the El Pinguico shaft has been suitably cleared, it is anticipated that crews will be able to access and sample the Colmillo Stope. This stope was a high grade area within the El Pinguico mine prior to its closure in 1913. Examples of historic sampling from this area, conducted in 1909, can be seen on page 5 of the Company's corporate presentation, available on its Website: www.vangoldmining.com.[3]
Geologist and Vangold Director, Bill Gehlen said "Historical assays from the Colmillo stope area are remarkably high-grade. We want to establish whether that material still exists, whether the grade from our sampling is comparable to historic grades, and whether other areas of high-grade are readily accessible within that part of the old workings. In the months ahead, we plan to test for extensions to these areas with an underground drilling campaign."
The El Pinguico Project:
El Pinguico is a high-grade gold and silver deposit that was mined from the early 1890s until 1913. The mining was done exclusively from the El Pinguico and El Carmen veins, which are thought to be splays off the Mother Vein, or ‘Veta Madre.'
The Veta Madre is associated with a mega fault that outcrops for 25 kilometres and is the most important source of precious metal mineralization in the region. The Veta Madre may cross Vangold's property at depth, underneath the high grade El Pinguico and El Carmen veins. Very limited drilling has been done on the property and no drilling has attempted to encounter the Veta Madre at depth.
Historic stockpiles of mineralized material exist on surface and underground at El Pinguico which may potentially provide feed to one of several operational mills in the Guanajuato area.
Hernan Dorado, a director of Vangold and a qualified person as defined by National Instrument 43-101, Standards of Disclosure for Mineral Projects, has approved the scientific and technical information contained in this news release.
About Vangold Mining Corp.
Vangold Mining is an exploration company engaged in the exploration of mineral projects in the Guanajuato region of central Mexico. The Company's flagship El Pinguico project is a significant past producer of high-grade gold and silver and is located just 7km south of the city of Guanajuato, Mexico. The Company remains focused on the near-term potential for development and monetization of both its surface and underground stockpiles of mineralized material from El Pinguico.
ON BEHALF OF THE BOARD OF DIRECTORS
"James Anderson"
Chairman and CEO
For further information regarding Vangold Mining Corp, please contact:
James Anderson, Director, +1 (778) 989-5346
Email: james@vangoldmining.com
Continue to watch our progress at: www.vangoldmining.com
Neither the TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Forward-Looking Statements
This news release contains certain forward-looking statements, which relate to future events or future performance (including, but not limited to, the potential for near term development and monetization of existing stockpiles of mineralized material at the Company's El Pinguico project in Mexico) and reflect management's current expectations and assumptions. Such forward-looking statements reflect management's current beliefs and are based on assumptions made by and information currently available to the Company. Readers are cautioned that these forward-looking statements are neither promises nor guarantees, and are subject to risks and uncertainties that may cause future results to differ materially from those expected including, but not limited to, market conditions, availability of financing, currency rate fluctuations, actual results of exploration and development activities, environmental risks, future prices of gold, silver and other metals, operating risks, accidents, labor issues, delays in obtaining governmental or regulatory approvals and permits, and other risks in the mining industry. In addition, there is uncertainty about the spread of the COVID-19 virus and the impact it will have on the Company's operations, global supply chains and economic activity in general. All the forward-looking statements made in this news release are qualified by these cautionary statements and those in our continuous disclosure filings available on SEDAR at www.sedar.com. These forward-looking statements are made as of the date hereof and the Company does not assume any obligation to update or revise them to reflect new events or circumstances save as required by applicable law.
VANGOLD MINING CORP.
PH: +1(778) 989-5346 E: info@vangoldmining.com W: vangoldmining.com
CA: #1000 – 409 Granville Street, Vancouver, British Columbia V6C 1T2, Canada
MX: Carr, Guanajuato – Silao km 5.5 Colonia Marfil Guanajuato, Mexico Office No.2 CP 36250
[1] This figure is historic in nature, has not been verified and should not be relied upon.
[2] VanGold completed 57 samples from 20 trenches (mostly historic with a few new trenches) at the top of the UG stockpile resulting in the average grades set out above. All samples were collected, recorded, bagged and sent by VanGold's consulting geologist to ALS Laboratory in Guadalajara, Mexico for sample preparation. Gold, silver and multi-element ICP analysis was completed at the ALS laboratory in North Vancouver, Canada. Rock samples were fine crushed (70% passing a 2mm screen), pulverized (85% passing a 75 micron screen) and a pulp split separated for assaying by a riffle splitter. 30 gram portion of each sample was assayed for gold by standard fire assay and a 10 gram split was analyzed for 35 elements by ICP method. Standard reference material and blank samples were inserted into the sample stream at a 5% insertion rate with pulped samples from the UG stockpile for quality control purposes. The results of the standards and blank samples were satisfactory. All data was collected with industry standard practices and assay results were verified by VanGold's consulting geologist. Further work by VanGold is required to verify the tonnage estimation by the Mexican Geological Survey agency and assess the distribution of grades within the UG stockpile.
[3] Historical assays have not been verified and should not be relied upon. They are presented as an indication of possible gold and silver mineralization within the Colmillo stope of the UG stockpile and as a guide for future work.
SOURCE: Vangold Mining Corp.
ReleaseID: 595040